Lithium cobalt oxide
| Lithium cobalt oxide[1] | |
|---|---|
|
lithium cobalt(III) oxide |
|
|
Other names
lithium cobaltite |
|
| Identifiers | |
| CAS number | 12190-79-3 |
| PubChem | 24867970 |
| Properties | |
| Molecular formula | LiCoO2 |
| Molar mass | 97.87 g mol−1 |
| Hazards | |
| Main hazards | harmful |
| (verify) (what is: /?) Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Lithium cobalt oxide (LiCoO2) is a chemical compound commonly used in the positive electrodes of lithium-ion batteries. The structure of LiCoO2 is known theoretically and has been confirmed with techniques like x-ray diffraction, electron microscopy, neutron powder diffraction, and EXAFS[2]: it consists of layers of lithium that lie between slabs of octahedra formed by cobalt and oxygen atoms.[3] The crystal structure is denoted R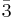 m[4] in Hermann-Mauguin notation, signifying a rhombus-like unit cell with threefold improper rotational symmetry and a mirror plane. More simply, however, both lithium and cobalt are octahedrally coordinated by oxygen. Each cobalt atom is aligned on a common axis with lithium atoms and separated from each lithium atom by a triangle of oxygen atoms as can be seen in the figures. The threefold rotational axis is termed improper because the oxygen triangles are anti-aligned.
m[4] in Hermann-Mauguin notation, signifying a rhombus-like unit cell with threefold improper rotational symmetry and a mirror plane. More simply, however, both lithium and cobalt are octahedrally coordinated by oxygen. Each cobalt atom is aligned on a common axis with lithium atoms and separated from each lithium atom by a triangle of oxygen atoms as can be seen in the figures. The threefold rotational axis is termed improper because the oxygen triangles are anti-aligned.
Exposure to soluble cobalt salts can lead to Beer Drinker's Cardiomyopathy.[5] MSDS sheets list lithium cobalt oxide is a potential human carcinogen[6][7] but indicate "no data available" under the Acute Toxicity heading.[8] However, unlike cobalt(II) salts, this oxide is insoluble in water. Lithium ion batteries contain lithium cobalt oxide and are considered nonhazardous waste.[9] Safety precautions should be taken when handling it.
The compound's usefulness as an intercalation electrode was discovered in 1980[10] by John B. Goodenough's research group at Oxford.
External links
References
- ^ Sigma-Aldrich product page
- ^ I. Nakai, K. Takahashi, Y. Shiraishi, T. Nakagome, F. Izumi, Y. Ishii, F. Nishikawa, T. Konishi (1997). "X-ray absorption fine structure and neutron diffraction analyses of de-intercalation behavior in the LiCoO2 and LiNiO2 systems". Journal of Power Sources 68 (2): 536–539. doi:10.1016/S0378-7753(97)02598-6.
- ^ Yang Shao-Horn, Laurence Croguennec, Claude Delmas, E. Chris Nelson and Michael A. O'Keefem (July 2003). "Atomic resolution of lithium ions in LiCoO2". Nature Materials 2 (7): 464–467. doi:10.1038/nmat922. PMID 12806387.
- ^ H. J. Orman and P. J. Wiseman (January 1984). "Cobalt(III) lithium oxide, CoLiO2: structure refinement by powder neutron diffraction". Acta Crystallographica Section C 40 (1): 12–14. doi:10.1107/S0108270184002833.
- ^ Donald G. Barceloux; Barceloux, Donald (1999). "Cobalt". Clinical Toxicology 37 (2): 201–216. doi:10.1081/CLT-100102420. http://informahealthcare.com/doi/abs/10.1081/CLT-100102420.
- ^ http://www2.produktinfo.conrad.com/datenblaetter/750000-774999/763023-da-01-en-AKKU_HANDY_NOKIA_3650_6600.pdf
- ^ http://www.swe.com/MSDS/BYD/MSDS_for_LP053048AH.pdf
- ^ http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do
- ^ http://www.ehso.com/ehshome/batteries.php#Summary
- ^ K. Mizushima, P.C. Jones, P.J. Wiseman, J.B. Goodenough (1980). "LixCoO2 (0<x<l): A NEW CATHODE MATERIAL FOR BATTERIES OF HIGH ENERGY DENSITY". Materials Research Bulletin 15: 783–789.
|
|||||||||||